Background
Ventricular tachycardia (VT) is a type of cardiac arrhythmia that affects over 300,000 Americans annually and is a leading cause of death. VT is characterized by episodes of rapid ventricular activation, often caused by reentrant electrical activity around and within areas of myocardial fibrosis and scar. VT patients have a decreased quality of life and increased risk of complications, including heart failure and sudden cardiac death.

Early forms of VT, where episodes occur infrequently, can be managed by antiarrhythmic medications. Though commonly, VT patients are non-responsive to medication and require alternate forms of intervention. Drug-refractory and/or high-risk VT patients often undergo implantation of a cardiac defibrillator (ICD), which can automatically deliver alleviative shocks during a VT episode. While life-saving, shock from an ICD can be traumatic and does not treat the underlying cause of the arrhythmia. Alternatively or in addition to ICD implantation, drug-refractory VT patients may undergo invasive ablation procedures. Catheter ablation (CA), the current gold standard method, uses a catheter guided intravascularly into the left ventricular chamber to deliver energy to disrupt and necrose areas of the ventricular myocardium responsible for the abnormal circuit. While successful in some cases, 30-60% of VT patients receiving CA result in recurrence. High recurrence rates are often associated with difficulty in ablating all potential arrhythmic pathways. Multiple VT pathways can exist throughout the depth of the ventricular myocardium, making transmural CA strategies challenging with physically constrained catheter systems. When traditional treatment fails, treatment-refractory VT patients are left with few options. There is a critical need to develop safer, more effective device and ablation therapies for treatment-refractory VT.
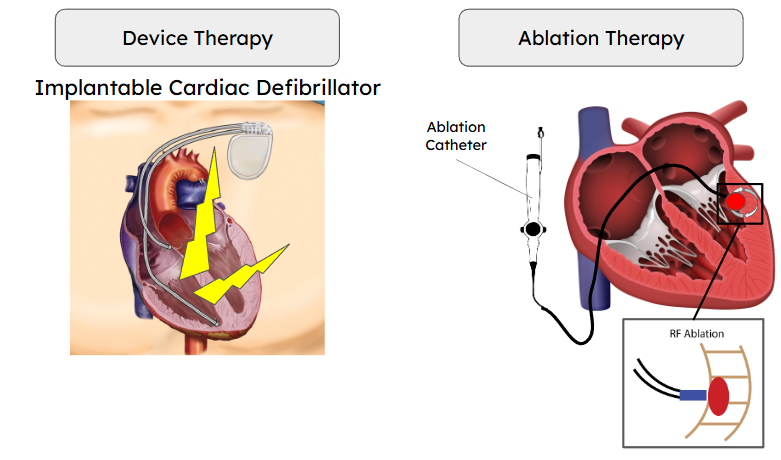
In the CEG, we are combining patient-specific computational modeling with experimental observations to investigate novel approaches in improving ICD function and ablation strategies.
Methods
Computational Modeling
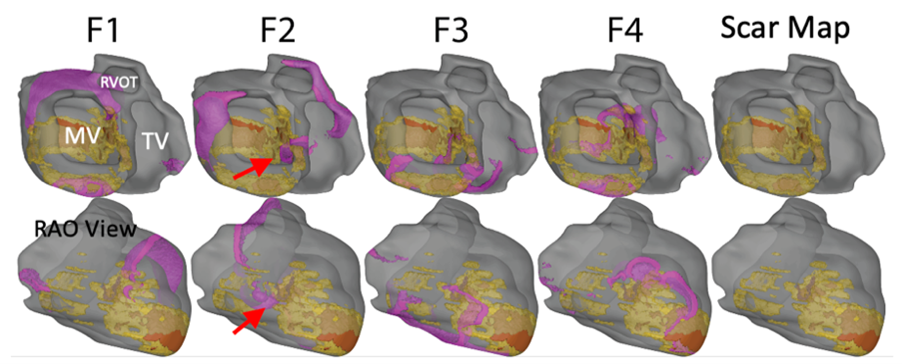
From medical imaging (MRI/CT), we can generate highly detailed models of cardiac electrophysiology (Check out the Digital Twins page for more information). We can then simulate ventricular tachycardia and characterize the circuit morphology. The figure below demonstrates VT wavefront propagation in one of our biventricular computational models, including scar and fibrosis information.
Experimental Investigation
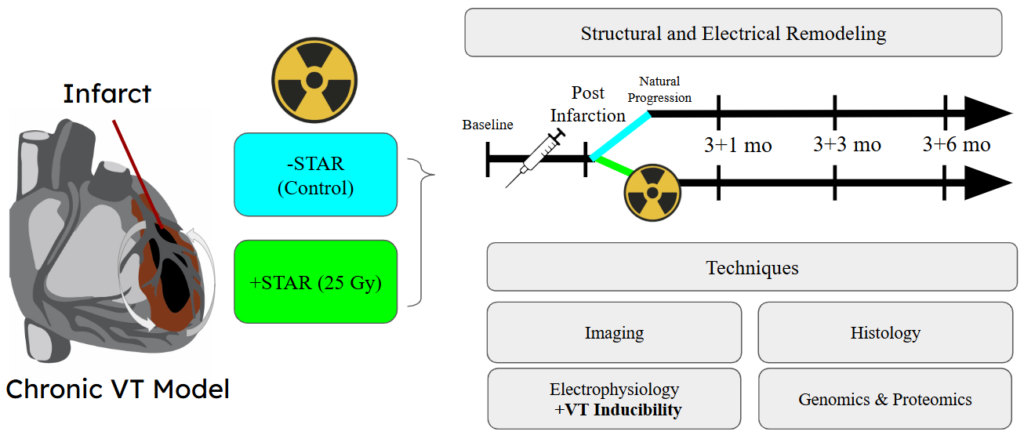
We also utilize pre-clinical experimental models to investigate novel therapies and mechanisms to help improve the clinical approach and treatment of VT. A newly developed non-invasive technique, stereotactic radiotherapy (STAR), uses external beams of energy to disrupt the underlying VT circuit and treat the arrhythmia. Our understanding of why STAR is an effective treatment remains limited. To enhance our understanding, we are investigating the time- and dose-dependent effects of STAR in both healthy and diseased preclinical models.
Relevant Papers
Paccione, Eric et al. “Radiation Induces Diffuse Extracellular Remodeling of Healthy Myocardium in a Dose and Time-Dependent Manner Without a Dense Ablative Effect” Heart Rhythm, 2025.

