Background
Atrial fibrillation (AF) is the most common cardiac arrhythmia, currently affecting between 3-5 million people in the US. This is expected to grow to over 12 million by 2030. When left untreated, AF leads to increased risk of stroke, heart failure, and other heart problems. AF is characterized by chaotic electrical signals in the atria (the upper chambers of the heart). These chaotic signals lead to dis-synchrony in contraction between the atria and ventricles (lower chambers of the heart).
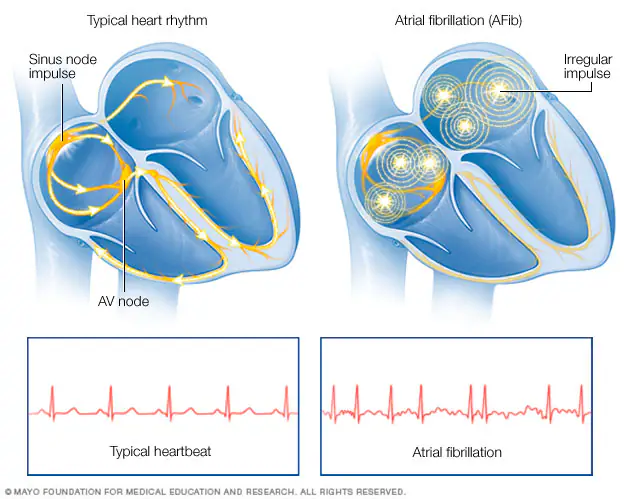
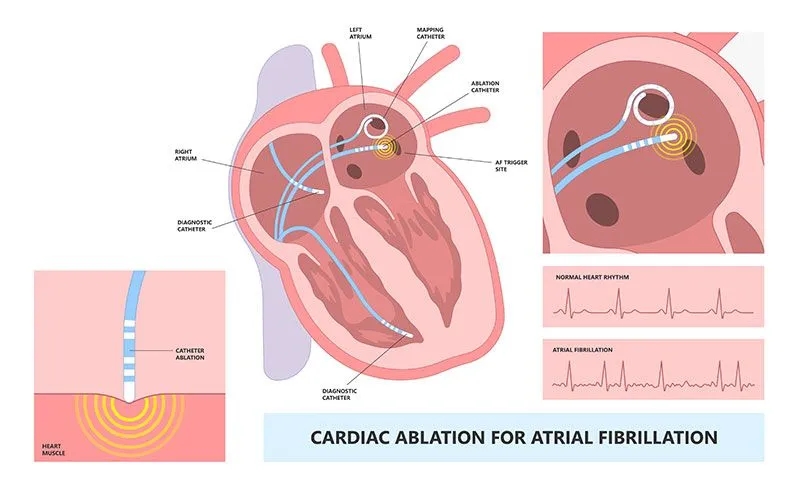
There are two main treatments for AF. The first is pharmacological treatments. These are drugs that aim to limit the rate and rhythm, preventing AF. When pharmacological treatments fail, surgical procedures are done to stop AF. The most common surgical procedure is cardiac ablation. During an ablation procedure, a doctor will steer a catheter inside the heart to deliver energy to kill tissue. Killing some of the cardiac tissue creates lines of scar, which prevent the chaotic signals from propagating through the atria.
Methods
Cardiac Digital Twins
In the CEG, we can leverage our computational tools and techniques to generate cardiac digital twins (CDTs) of a patient’s heart to use for pre-ablation planning (See the cardiac digital twins page for more details on this process). We can simulate AF in the CDT, helping guide the doctor to what areas may be causing the chaotic electrical signals. We can also test a variety of ablation patterns prior to the procedure. After applying a simulated ablation, we can then try and induce AF in the model once again, testing if an ablation pattern would prevent AF in a specific patient. These computationally guided approaches help to personalize the treatment of AF.

Cardiac Mapping
Another method we use to study AF is cardiac mapping. Cardiac mapping is performed by navigating a catheter with electrodes throughout the heart and collecting signals. These signals can be used to inform the doctor about the electrical activity in that area. In the CEG, we use high-density cardiac mapping in preclinical models to understand the mechanisms behind AF development and maintenance. With these models, we can study the progression of AF longitudinally, using mapping to track the electrical remodeling that occurs during disease progression.

Relevant Papers
Other papers…

